
There are many websites that claim that mixing vinegar and baking soda together in a spray bottle creates the most potent and safe household cleaner known to human kind. All you need is these two cheap ingredients and the eraser of all grime is as your fingertips. Unfortunately, most of us learnt the truth of these claims in elementary school but forgot.
Admittedly, this is one of the less nefarious instances of misleading information. The ingredients aren’t expensive, no one is selling anything (but page views), and it certainly does make some tasty chips. It unfortunately does show that the way chemistry is taught to most students (at least here in the US) does not appear to stick. While there are plenty of “Mitochondria is the powerhouse of the cell” memes, scientific education is important!
I am not going to claim that I am a chemistry expert at all. I was previously a member of the American Chemical Society, so I do know some things – mostly how to find out things that I don’t know yet! And that’s the most important skill to have. I did a lot of re-learning and research for this “simple” factoid.
And if you are not aware, I would like to show you something. This is how vinegar and baking soda neutralize each other and form salt water. Yes, that means that you are cleaning with smelly salt water. Tasty, smelly, sodium acetate salt water that makes delicious chips. (Anyone else wonder how vinegar salt would taste on green beans?)
Vinegar
Vinegar, or store-bought white vinegar, is 95% water (H2O) and 5% Acetic Acid (HC2H3O2 or CH3COOH, it is all the same – two carbon atoms, four hydrogen atoms, and two oxygen atoms) [2]. Acetic acid is the active ingredient in all types of vinegar. When apple cider vinegar, red wine vinegar, and other flavored vinegars are brewed, acetic acid is created during the fermentation process – or at least, something similar to the fermentation process. These other vinegars may contain other plant compounds (like apple cider and wine), but the chemical reaction only occurs on the acetic acid [3].
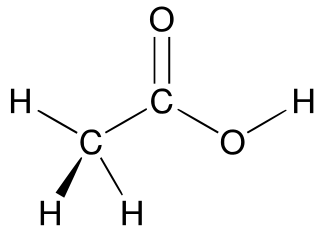
Acetic Acid (Vinegar) Chemical Structure via Wikimedia
All by itself, vinegar is antifungal, antibacterial, and may help treat ear infections, according to PubChem. It is often found in store bought cleaners, and works like a solvent and to increase lipid solubility – that means dissolving the grease on your stove. Vinegar is a very strong acid, and will typically have a pH around 2.5 [2]. Baking soda, by contrast, is a weak base, with a pH of 8 to 9 [4].
Baking Soda
The popular leavening agent baking soda is also known as Sodium Bicarbonate, or NaHCO3. It is comprised of sodium, hydrogen, carbon, and oxygen. The positively charged sodium atom is attracted to the negatively charged bicarbonate [4]. Baking soda is both manufactured as well as mined from the ground. Sodium bicarbonate can take the form of a white crystal called Nahcolite. It can be found on every continent minus Australia and Anarctica [6].
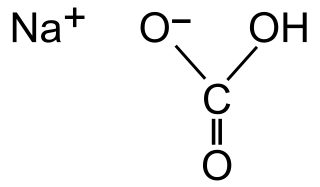
Sodium Bicarbonate (Baking Soda) Chemical Structure via Wikimedia
Baking Soda is derived from Sodium Carbonate, which was used as early as 3500 BCE by the ancient Egyptians [7]. The first use of it in bread making is attributed to Native American / American Indians use of pearl ash (or potash, potassium carbonate). This method of bread leavening was taught to the early American colonists, who in turn, spread this information back to Europe [8]. Europeans used this method with natron, or natural soda ash (sodium carbonate), and it later became popularized as Irish Soda Bread. In 1791, sodium bicarbonate was chemically derived from soda ash. By 1846, it was a household item [7].
Baking soda is useful in many different applications and industries aside from modern baked goods. Medicinally, baking soda has been used most commonly as an antacid, in both the gastrointestinal sense and in most toothpastes. It also has applications in kidney disease, sickle cell anemia, metabolic acidosis, and post surgical needs [4].
Things Get Bubbly
When the acetic acid in vinegar mixes with basic sodium bicarbonate, a double displacement reaction occurs [9]. A double displacement reaction occurs when the positively and negatively charged ions trade place.
Sodium Bicarbonate + Acetic Acid = NaHCO3 + HC2H3O2
Baking Soda (NaHCO3) is comprised of positive Na and negative HCO3.
Vinegar’s acetic acid contains positive H and negative C2H3O2.
These four components switch places:
NaHCO3 + HC2H3O2 → NaC2H3O2 + H2CO3
The positive Na from the baking soda attaches to the negative C2H3O2 from the vinegar, forming sodium acetate (NaC2H3O2).
The positive H from the vinegar attaches to the negative HCO3 from the baking soda. This forms carbonic acid (H2CO3).
Carbonic acid is not stable, and immediately breaks down into water (H2O) and carbon dioxide (CO2) [10]. This secondary reaction is called a decomposition reaction [9].
H2CO3 → H2O + CO2
Finally, the result can be summed up as thus:
NaHCO3 + CH3COOH → NaC2H3O2 + H2O + CO2
The water mixes with the rest of the water in the vinegar, and the carbon dioxide bubbles up and is released as a gas [10]. In the traditional volcano science experiment, dish soap and food coloring are added. The dish soap encapsulates the carbon dioxide bubbles so that they do not pop, and “explode” out of its container instead of being released [1].
Sodium Acetate
Sodium acetate is an organic sodium salt which is used both in medicine as an electrolyte as well as in industrial applications as . When mixed with more acetic acid to create sodium diacetate, the salt and vinegar chip flavoring. It is a salt, and has many of the same properties of table salt, but with a vinegar flavor [5].
These properties mean that the clean like table salt, as well.
And Now to Burst Your Bubble
Cleaning with vinegar and baking soda after the reaction has taken place is cleaning with salt water. Shaking these ingredients up in a spray bottle will neutralize them and render them as ineffective cleaning agents. Any cleaning ability is derived from the salt as an abrasive agent, or any leftover primary ingredients.
Now alone, these ingredients are very useful, and they can be used in tandem. For example, you can clean with vinegar, and then neutralize the smell afterward with baking soda and water. You can clean an appliance with baking soda and then rinse any residue with vinegar. These ingredients can work together, but their uses become limited once the chemical reaction takes place.
Alternatively, you can always make some chips or a mini volcano!
Do you like WildHemlock.Com?
Support with Paypal!
Sources:
- Deziel, Chris. “Vinegar Experiment for Endothermic and Exothermic Reactions.” Sciencing, Leaf Group Media, 26 Apr. 2018, <https://sciencing.com/vinegar-experiment-endothermic-exothermic-reactions-18163.html>
- National Center for Biotechnology Information. PubChem Database. Acetic acid, CID=176, <https://pubchem.ncbi.nlm.nih.gov/compound/Acetic-acid> (accessed on Feb. 20, 2020)
- Johnston, Carol S, and Cindy A Gaas. “Vinegar: medicinal uses and antiglycemic effect.” MedGenMed : Medscape general medicine vol. 8,2 61. 30 May. 2006 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1785201/>
- National Center for Biotechnology Information. PubChem Database. Sodium bicarbonate, CID=516892, <https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-bicarbonate> (accessed on Feb. 20, 2020)
- National Center for Biotechnology Information. PubChem Database. Sodium acetate, CID=517045, <https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-acetate> (accessed on Feb. 20, 2020)
- “Nahcolite: Mineral Information, Data and Localities“, MinDat.Org, Hudson Institute of Minerology, <https://www.mindat.org/min-2831.html>
- Waple, Brian. “Sodium bicarbonate.” Restoration 2 (2017). <https://www.armex.com/sites/armex.com/files/resources/Sodium%20Bicarb%20the%20User-Freindly%20Blasting%20Abrasive.pdf> Warning, PDF!
- O’Dwyer, Edward J. History of Soda Bread. Society for the Preservation of Irish Soda Bread, 2003, <http://www.sodabread.info/history>
- Helmenstine, Anne Marie. “Know the Equation for the Baking Soda and Vinegar Reaction.” ThoughtCo, 31 Jan. 2020, <https://www.thoughtco.com/equation-for-the-reaction-of-baking-soda-and-vinegar-604043>
- Senese, Fred. “How Should the Reaction between Vinegar and Baking Soda Be Classified?” General Chemistry Online, Frostburg State University, <https://antoine.frostburg.edu/chem/senese/101/reactions/faq/classify-vinegar-bakingsoda.shtml>

